spectroscopy - Absorption and emission at same wavelength? - Chemistry Stack Exchange
$ 10.99 · 4.5 (171) · In stock

Is it possible for a molecule to absorb and emit at the same wavelength? What is the reason behind it? I’m working on charged tin porphyrins and got the excitation and emission (fluorescence) wavel

/cms/asset/e7d3fe9d-08b7-4dd

Emission Spectra Light Wavelength Deviations for Mercury

analytical chemistry - What is the role of a monochromator in

solid state chemistry - What is the principle of diffuse

quantum chemistry - How can Planck's equation and the wave

atomic structure - Relation of Energy required to change an orbit

molecular orbital theory - A molecule absorbs light having a

spectroscopy - What is the region of this wavelength (462 nm

how Violet's Wavelength is 766.5? : r/JEENEETards

mass spectrometry - Difference between an absorbance, emission

spectroscopy - Conversion from Spectral Line Intensity to

photochemistry - Is it possible for a substance to absorb a longer

analytical chemistry - Why should AAS use element lamps

atoms - How to interpret a luminescence intensity vs wavelength
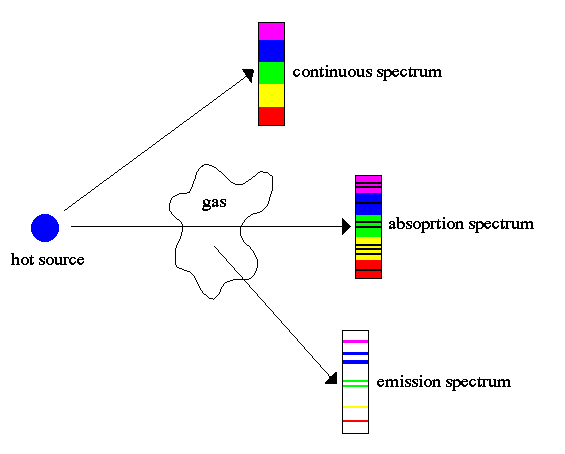
Absorption and Emission Lines